- Carbon Atomic Mass 12 Amu
- Carbon Atomic Mass With Units
- Carbon Atomic Mass Rounded
- Carbon Atomic Mass G/mol
Molar mass of C = 12.0107 g/mol

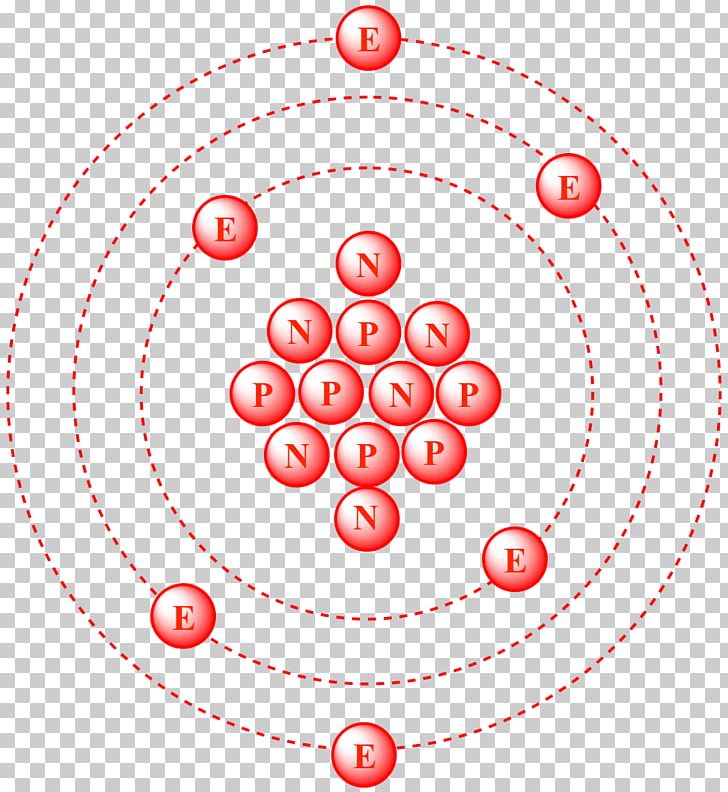
Carbon is present in the 14th group of elements in the Periodic Table. The atomic number of carbon is 6 and the atomic mass is 12.01gmol-1. It is represented by the symbol C. This picture explains the idea of 'atomic mass'. The carbon atom (14 C) nucleus on the top has 6 protons plus 8 neutrons.It has an atomic mass of 14. Tritium (3 H), an isotope of hydrogen, is shown on the bottom.It has 1 proton plus 2 neutrons in its nucleus.
Convert grams Carbon to moles or moles Carbon to grams
| Symbol | # of Atoms | Carbon | C | 12.0107 | 1 | 100.000% |

In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together.
Finding molar mass starts with units of grams per mole (g/mol). When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. Futura fonts for mac. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.
Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance.
A common request on this site is to convert grams to moles. To complete this calculation, you have to know what substance you are trying to convert. The reason is that the molar mass of the substance affects the conversion. This site explains how to find molar mass.
Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. These relative weights computed from the chemical equation are sometimes called equation weights.
If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom (or group of atoms) in the formula by the formula weight and multiplying by 100.

The atomic weights used on this site come from NIST, the National Institute of Standards and Technology. We use the most common isotopes. This is how to calculate molar mass (average molecular weight), which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass.
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 12C | 12(exact) | [0.9884, 0.9904] |
| 13C | 13.003 354 835(2) | [0.0096, 0.0116] |
The 12C isotope has served since 1960 as the scale-determining reference for the definition of theunified atomic mass unit and is the basis of all atomic weights. The zero value for the delta scale usedin relative isotope-ratio measurements of carbon since the 1950s was based on a sample of fossil marine carbonate(Belemnitella Americana, Peedee Formation, Cretaceous Period, South Carolina, also known as PDB).
In 1961, the Commission recommended Ar(C) = 12.011 15(5) and in 1969 it recommended Ar(C) = 12.011(1).The larger uncertainty was assigned to include all terrestrial sources of carbon whose isotopic compositions had been measured tothat time. After the supply of PDB was exhausted, a modified delta scale was recommended for relativecarbon isotope-ratio measurements (referred to as the Vienna PDB, or VPDB scale) that yields the samezero value as the PDB scale when based on measurements of a new carbonate reference material knownas NBS 19. In 1995, the Commission recommended Ar(C) = 12.0107(8) as a result of a re-evaluationof variations in normal terrestrial materials.
Variations in the n(13C)/n(12C) ratio of terrestrial sources of carbon are caused largely by biogeochemicalreactions and physical processes. Some of the largest effects are associated with oxidation-reductionreactions including photosynthesis, such that organic substances and reduced natural gases typicallyare depleted in 13C relative to carbonate materials and the atmosphere. Differences in the degreeof 13C depletion during photosynthesis are characteristic of some groups of plants and may be passedalong to plant consumers, such that carbon isotope studies can be used to identify features of animal dietsand paleoclimates. Variations in the relative rates of organic carbon production, burial, and oxidation throughgeologic time are recorded in the isotopic compositions of sedimentary rocks. The highest reported 13C abundance is from dissolved carbonate in reduced marine sediment pore water with x(13C) = 0.011 466 andAr(C) = 12.011 50. The lowest reported 13C abundance is from crocetane recovered from the ocean bottom at cold seeps in the northern Pacific Ocean with x(13C) = 0.009 629 and Ar(C) = 12.009 66.
Carbon Atomic Mass 12 Amu
The radioactive 14C isotope has a half-life of 5730 a. It is introduced continuously to the near-surfaceenvironment of the earth by cosmic-ray reactions, from cosmic dust, and by nuclear technology. Itis of great interest for prehistoric dating as well as archaeological, anthropological, paleotemperature,and zoological studies. Yet, this isotope never occurs in normal carbon sources in concentrations high enoughto affect significantly the Ar(C) value. Before nuclear weapons tests, the abundance of 14C in the atmospherehad an average value of only about 10−16. It should be noted that a half-life of 5568 a (theso-called 'Libby half-life'), has been adopted by convention for calculations in geochronology.
Carbon Atomic Mass With Units
Atomic weights of the elements 2009 by M.E. Wieser and T.B. Coplen. Pure Appl. Chem. 2011 (83) 359-396
CIAAW
Carbon
Ar(C) = [12.0096, 12.0116] since 2009
The name derives from the Latin carbo for 'charcoal'. It was known in prehistoric times in the form ofcharcoal and soot. In 1797, the English chemist Smithson Tennant proved that diamond is pure carbon.
Carbon Atomic Mass Rounded
Natural variations of carbon isotopic composition
Carbon Atomic Mass G/mol
Isotopic reference materials of carbon.
